Mapping the cell and gene therapy landscape: community insights
Cell & Gene Therapy Insights 2025; 11(6), 797–801
DOI: 10.18609/cgti.2025.089
 |
Over a six-week period in May 2025, we surveyed the Cell and Gene Therapy Insights community on LinkedIn to evaluate the current state of the cell and gene therapy (CGT) field—exploring current challenges, emerging technologies, and expectations for the future. With 950 total responses, the results revealed key pain points, major barriers to progress, and the most promising innovations expected to drive the field forward.
The key challenges in the CGT field
As the number of CGT products approved by regulatory agencies such as the US FDA and the EMA continues to rise each year, demand for these therapies has grown substantially. This surge emphasizes the urgent need for manufacturers to innovate and streamline their production processes to keep up with the rapid evolution of the CGT landscape. However, several challenges must be addressed to alleviate the clinical translation of CGTs and increase patient access.
What is the biggest bottleneck in cell and gene therapy manufacturing today? | 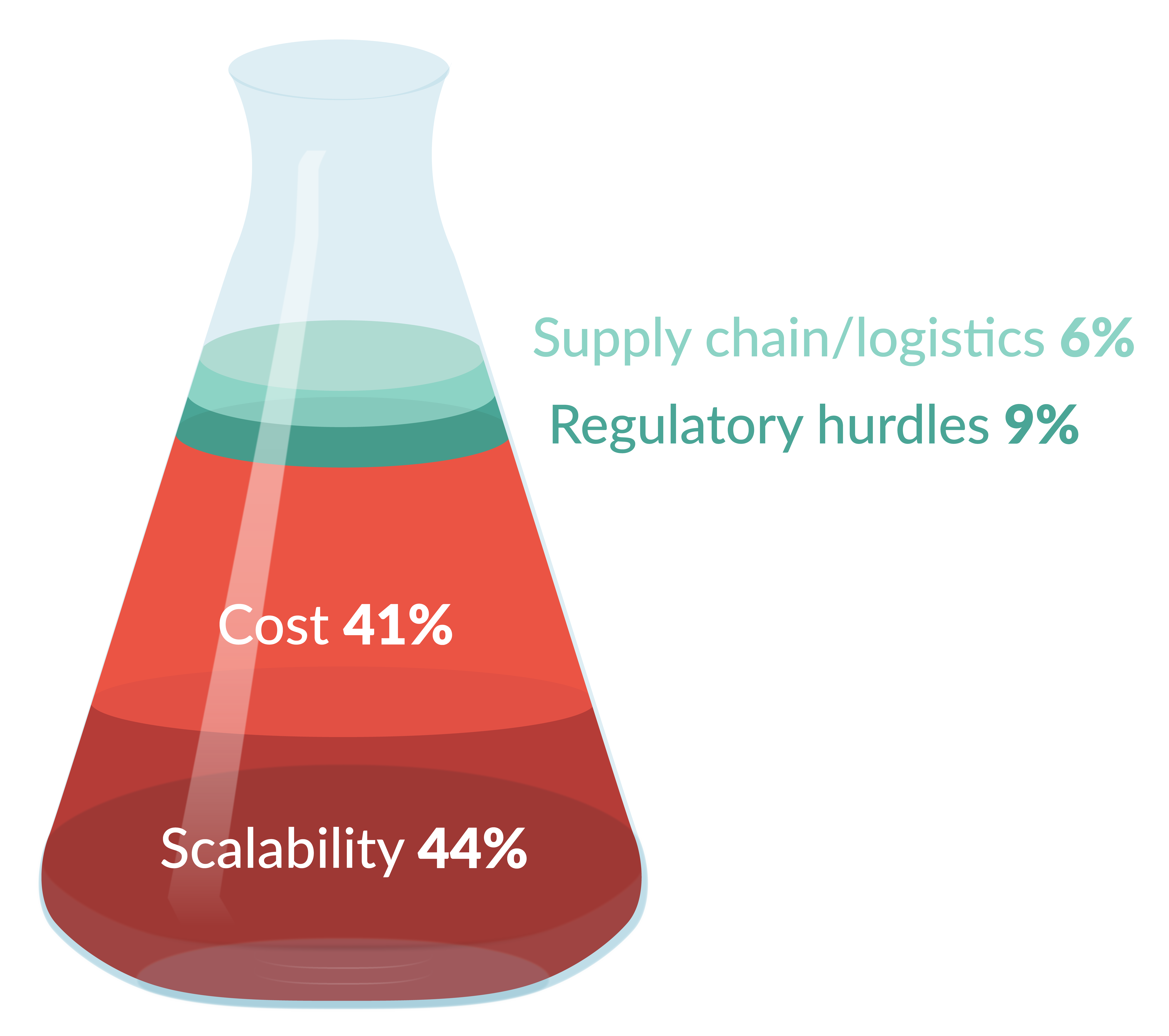 |
According to our polls, 44% of respondents see scalability as the biggest bottleneck in CGT manufacturing, closely followed by cost at 41%. While supply chain (6%) and regulatory challenges (9%) are still concerns, they are viewed as less significant in comparison.
Regarding patient access, the high cost of CGTs is still a major barrier, which raises discussions around affordability, reimbursement, and long-term value.
Excluding cost, what is the most significant barrier to ensuring global patient access to cell and gene therapies?
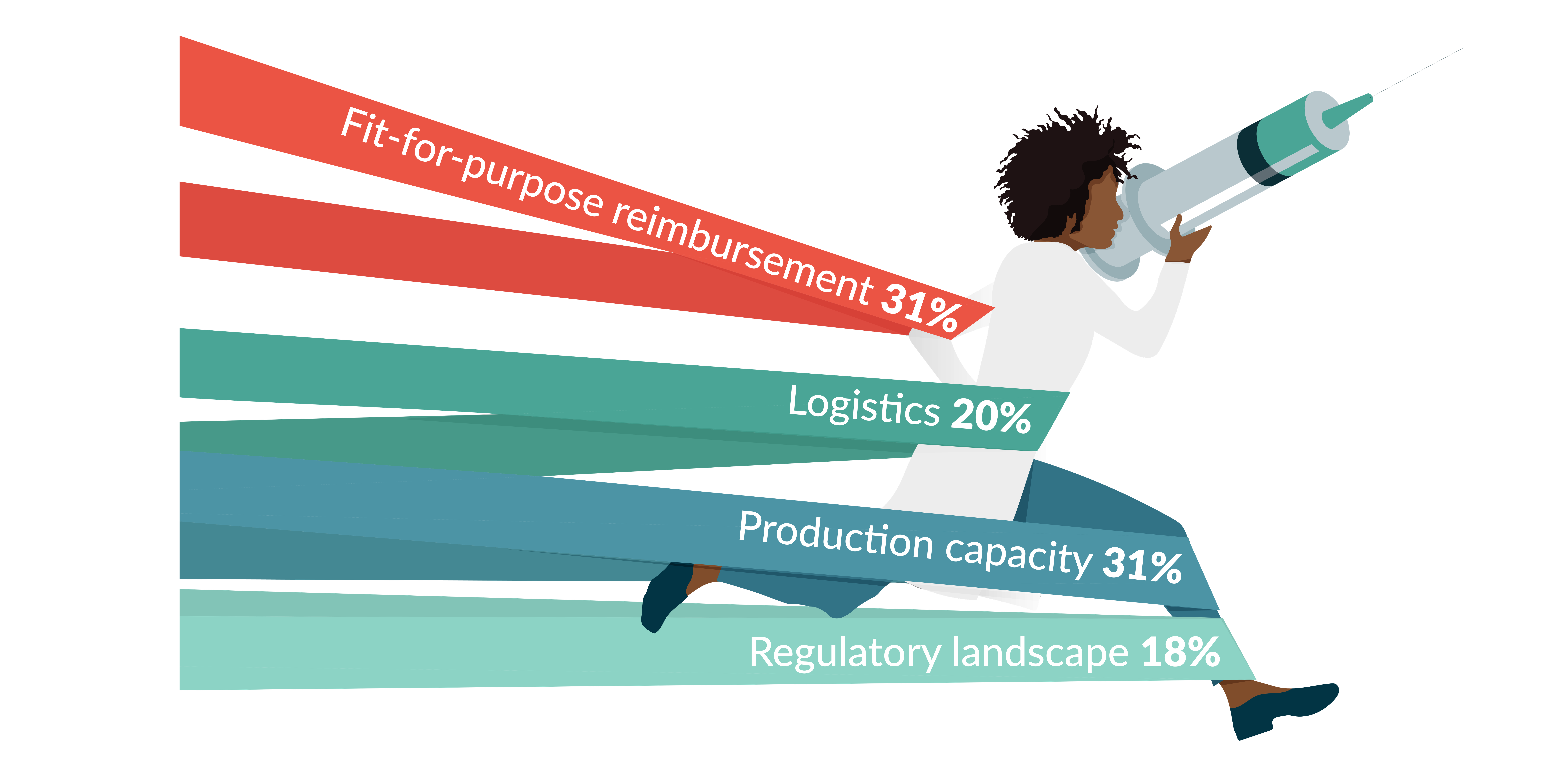 |
Apart from cost, our community identified fit-for-purpose reimbursement models and limited production capacity as equally pressing challenges (both at 31%). Logistics (20%) and regulatory hurdles (18%) also continue to pose hurdles to global access.
What represents the greatest threat to the cell and gene therapy sector in the coming 5 years?
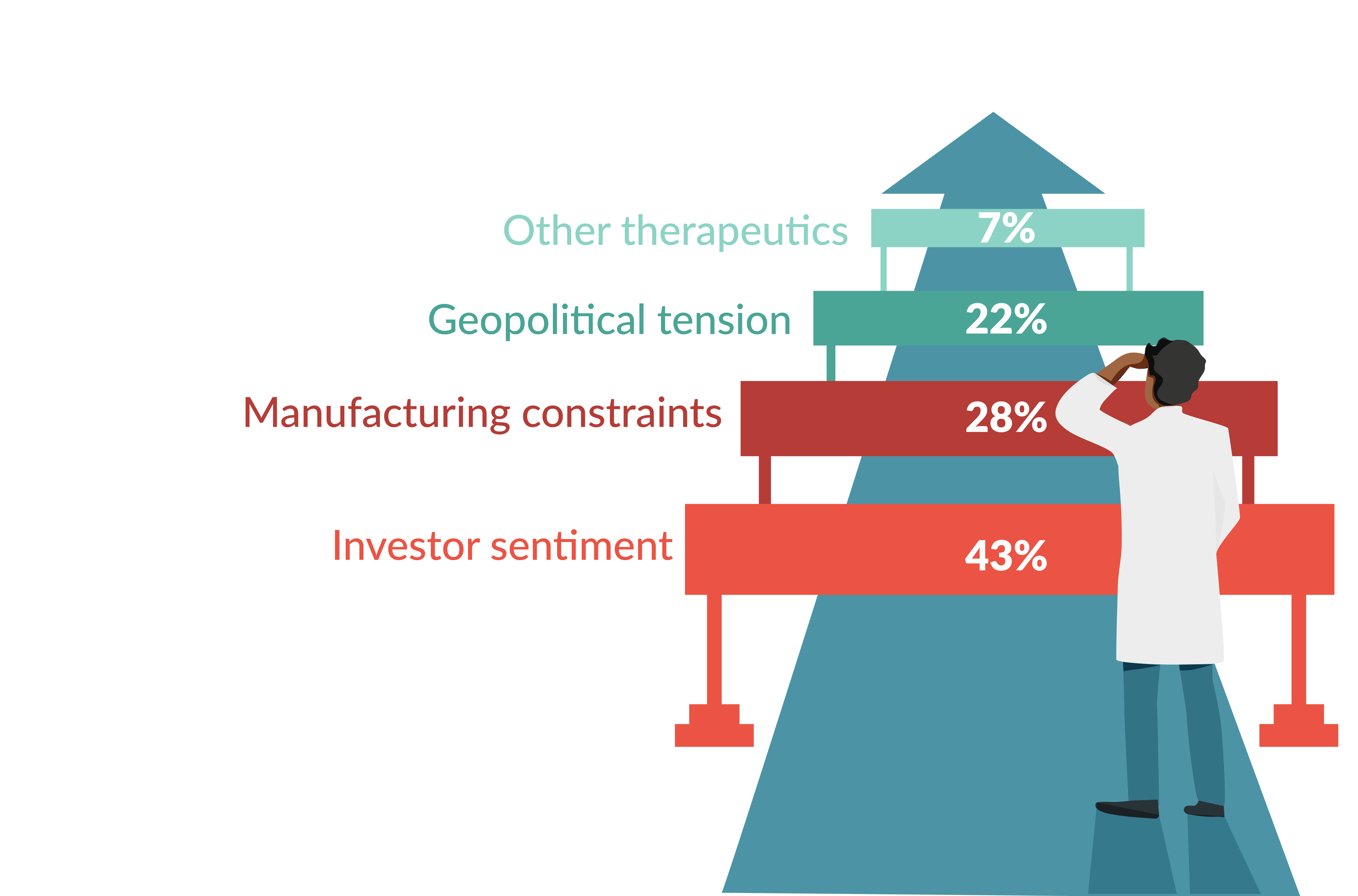
Furthermore, our polls highlight investor sentiment as the most significant perceived threat to the CGT sector over the next five years, cited by 43% of respondents. Manufacturing constraints also pose a major concern, accounting for 28% of responses, while geopolitical tensions were noted by 22%. Comparatively, competition from other therapeutic modalities was viewed as a lesser risk, identified by only 7%. These findings suggest that financial confidence and scalable production capabilities are seen as critical challenges for the future growth of CGT.
Exploring novel technologies
The emergence and maturation of novel technologies are transforming both the development and delivery of CGTs, including non-viral genetic material delivery tools such as lipid nanoparticles (LNPs), as well as the rise of AI. Together, these technologies are helping to overcome longstanding challenges in CGT, creating space for more effective, scalable, and personalized therapies.
Which gene delivery technology area will witness the greatest innovation over the next 5 years?
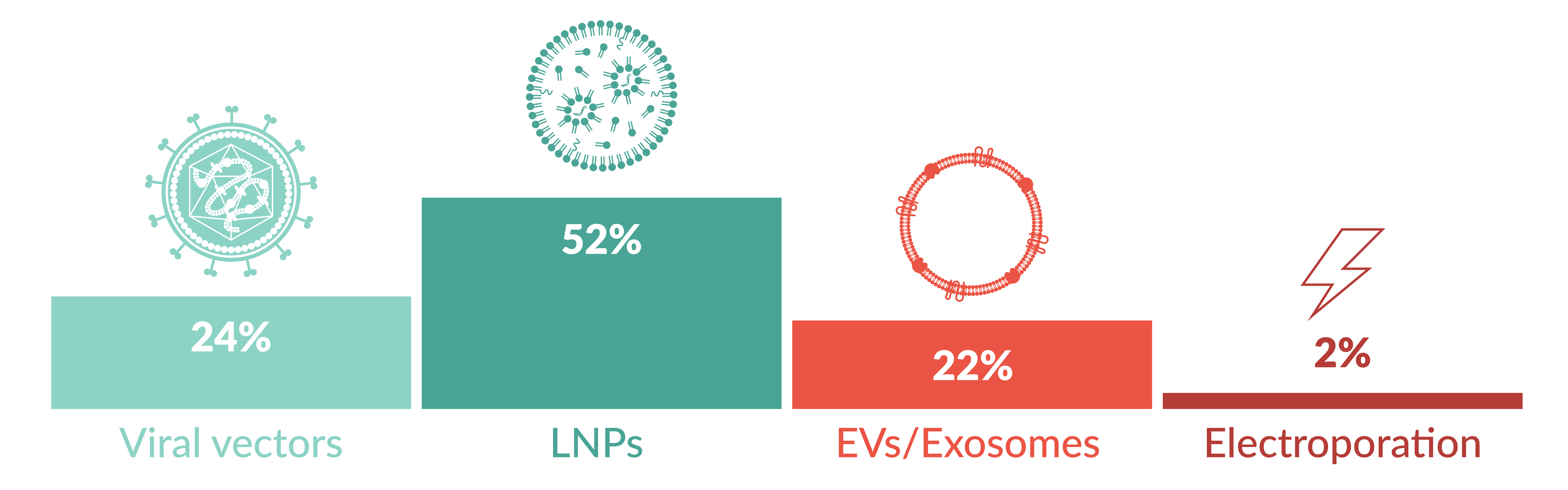 |
Our survey results indicate that LNPs are expected to drive the most innovation in gene delivery over the next five years, with 52% of respondents selecting them as the leading area of advancement. Viral vectors and extracellular vesicles/exosomes followed at 24% and 22% respectively, reflecting continued interest in these platforms. Electroporation, by contrast, was seen as having limited innovation potential—only 2% of respondents highlighted it.
Can AI/machine learning technologies revolutionize cell and gene therapy manufacturing?
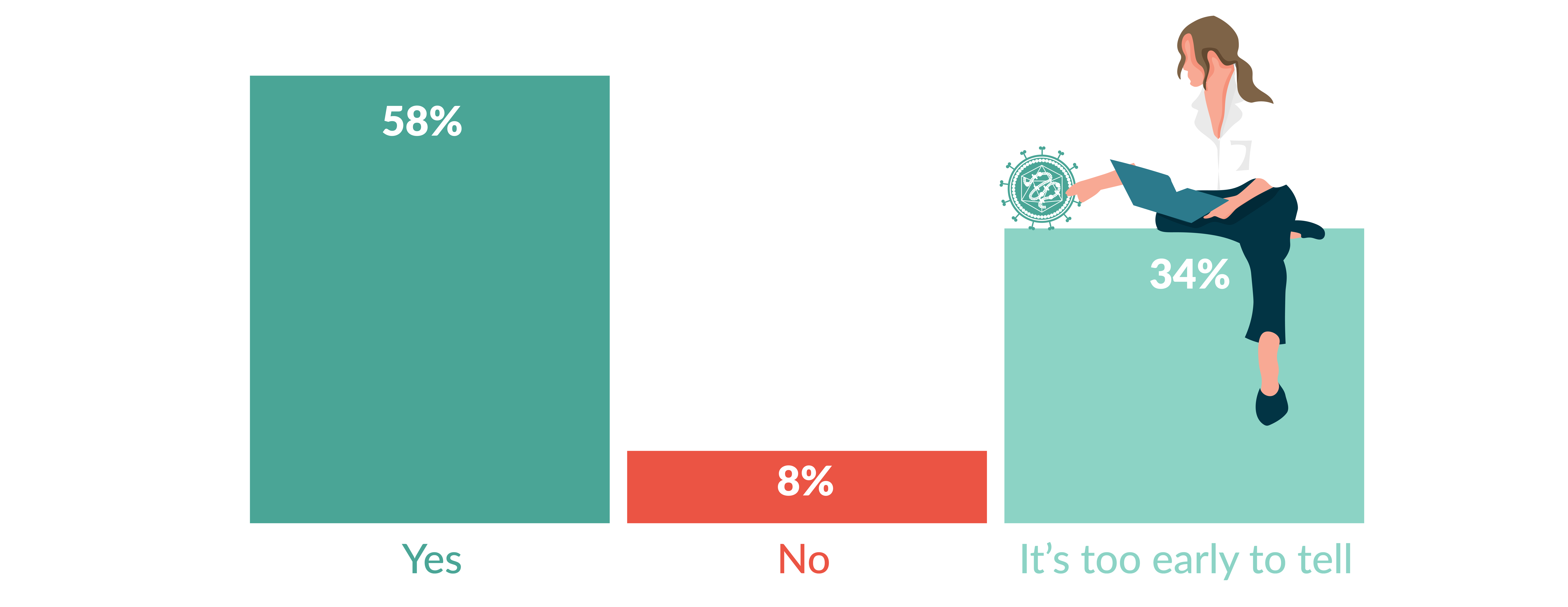 |
When it comes to AI and ML, most respondents (58%) believe that these technologies have the potential to revolutionize CGT manufacturing. While some remain uncertain or skeptical, the prevailing sentiment suggests strong optimism around the role of advanced data analytics and automation in streamlining production, improving quality control, and accelerating process development in the CGT sector.
Exploring the future direction of the CGT sector
 |
While many challenges are yet to be addressed, the CGT sector is rapidly advancing and showing tremendous promise for the future of medicine. With 43 CGT products now approved by the FDA, the field is transitioning from experimental treatments to commercially viable therapies.
Do you see gene therapies becoming part of routine treatment for conditions like hemophilia and muscular dystrophy in the next few years? | 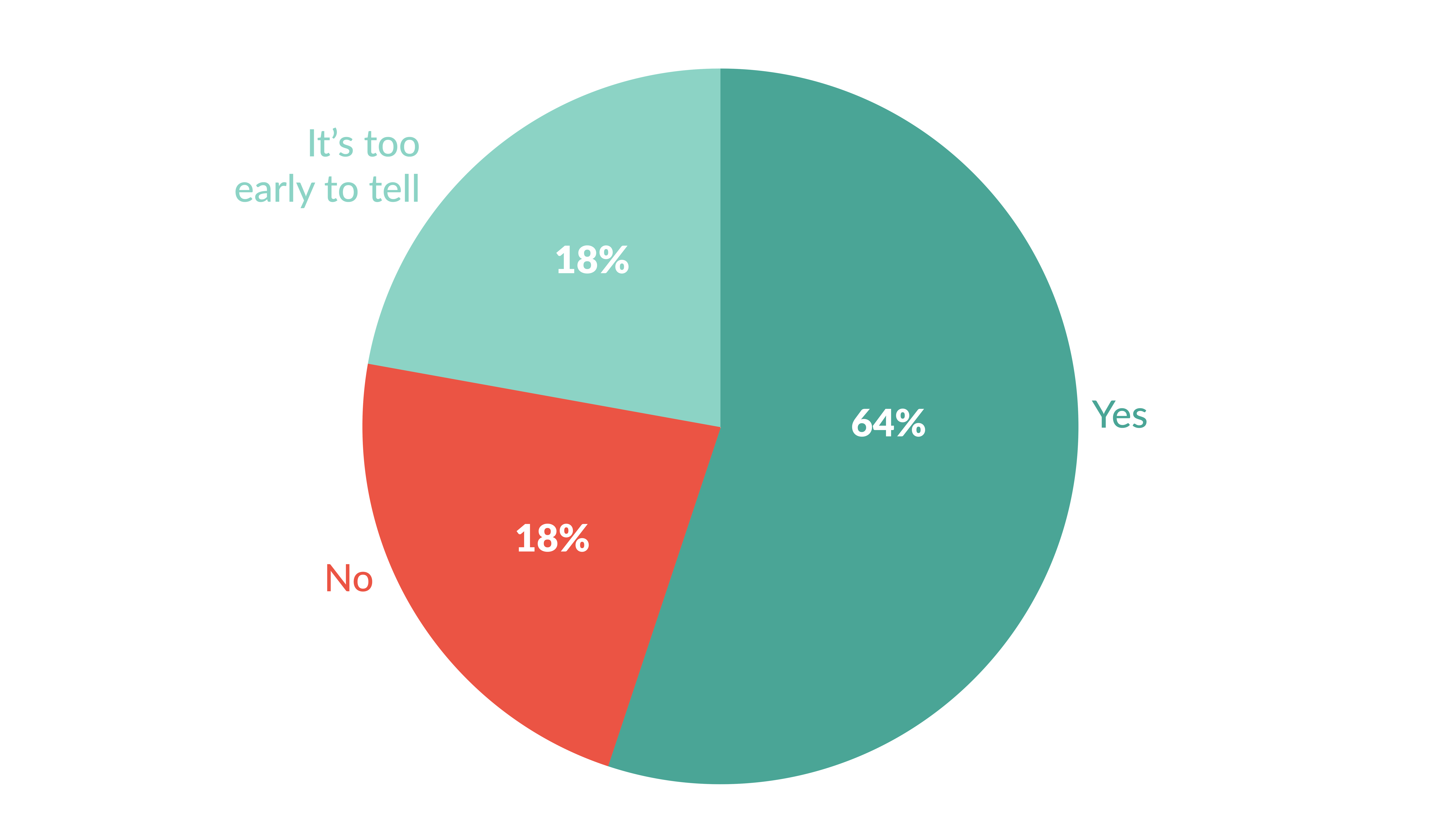 |
For example, nearly two-thirds (64%) of our respondents believe gene therapies will become part of routine treatment for conditions like hemophilia and muscular dystrophy in the coming years.
Which “future of cell and gene therapy” topic are you most curious about?
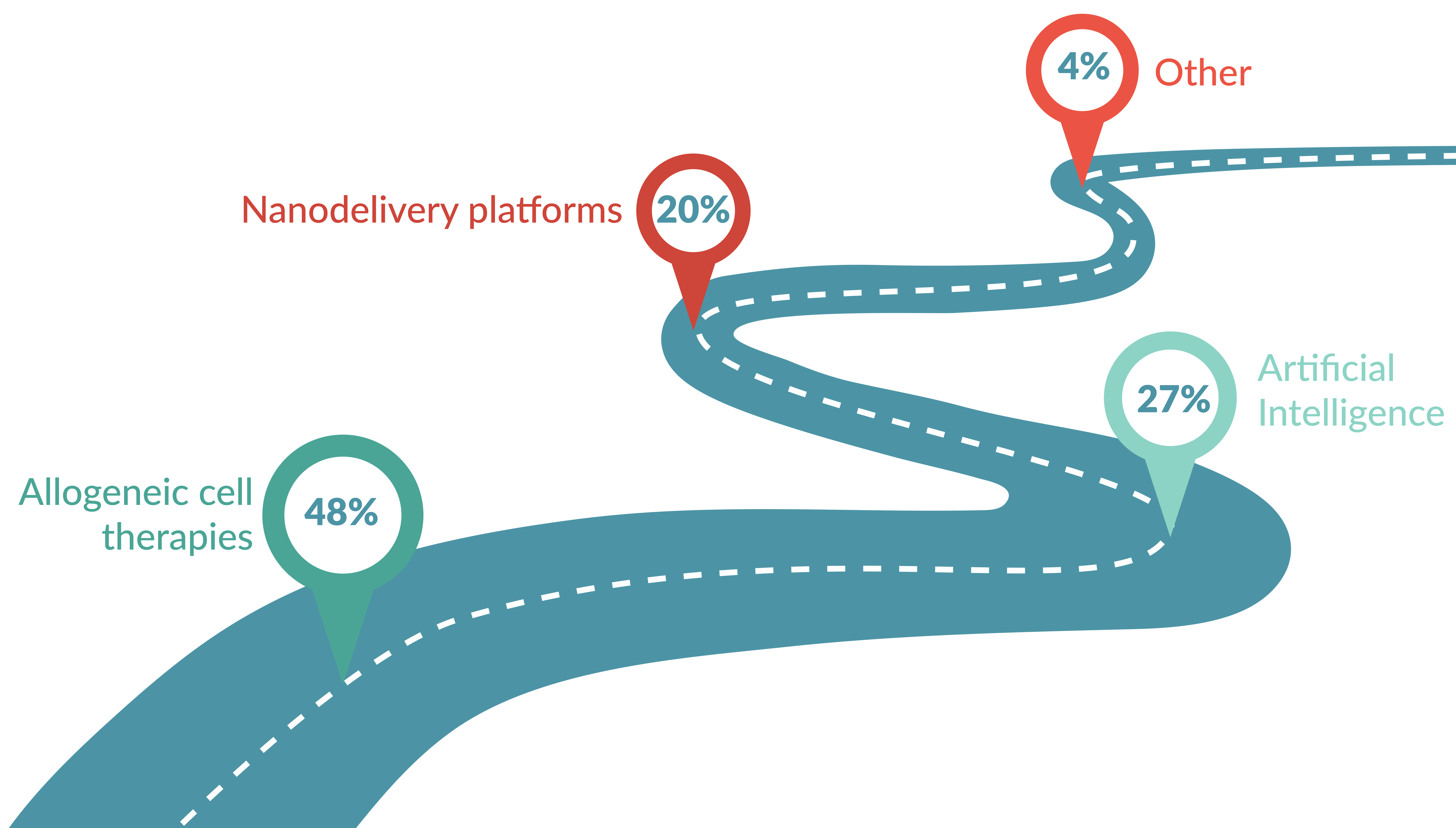 |
Continued innovation in areas such as gene delivery, manufacturing, and data-driven development is paving the way for broader access, improved efficacy, and expanded indications across a range of diseases.
When asked which future topic in CGT they’re most curious about, 48% of respondents chose allogeneic cell therapies, followed by artificial intelligence (27%) and nanodelivery platforms (20%)—highlighting strong interest in scalable, next generation treatment approaches.