From bench to bedside: navigating bioanalytical method development and validation for clinical efficacy and regulatory compliance
Cell & Gene Therapy Insights 2025; 11(8), 851–865
DOI: 10.18609/cgti.2025.098
Bioanalytical methods are indispensable in pharmaceutical development, serving as the backbone for ensuring clinical efficacy and regulatory compliance. The development and validation of these methods face numerous challenges for improved clarity and flow, as recommended. Biological matrices such as blood, plasma, urine, and tissues often contain endogenous substances that interfere with analyte detection, complicating efforts to achieve accuracy and reliability. These challenges highlight the need for innovative strategies to optimize bioanalytical methods for both clinical and regulatory success.
Biological matrices, ranging from those encountered in small molecule studies to complex biologics, including cell and gene therapies, present inherent complexities due to endogenous biomolecules that can interfere with accurate detection and quantification of analytes, such as therapeutic vectors or transgene products. Effective sample preparation, matrix-matched calibration, and advanced detection techniques are essential to mitigate matrix effects and enhance sensitivity. Achieving high selectivity is especially critical in early-phase clinical studies and biomarker analysis. Advanced instrumentation, derivatization techniques, and multiple reaction monitoring (MRM) are pivotal for precise quantification and reliable results. Adherence to stringent guidelines from regulatory bodies such as the US FDA and EMA requires thorough method validation, meticulous documentation, and consistent implementation of regulatory standards. Stability studies are essential to address challenges related to analyte degradation. Techniques such as stabilization methods and rapid processing are critical to maintaining analyte integrity throughout the analytical process. Developing bioanalytical methods is resource-intensive, requiring efficient cost management, strategic planning, and where appropriate, collaboration with academic institutions and contract research organizations (CROs) to optimize processes.
Bioanalytical methods are essential for advancing pharmaceutical development from bench to bedside, providing the accuracy and reliability needed to support clinical efficacy and regulatory compliance. Key challenges—including matrix complexity, limited sensitivity, analyte instability, and stringent regulatory expectations—demand robust and well-validated methods. To address these issues, laboratories are increasingly leveraging advanced strategies such as optimized sample preparation techniques, matrix-matched calibration, and MRM. These approaches help mitigate matrix effects, improve detection sensitivity, and ensure analyte stability across diverse biological matrices. By integrating such practical and innovative solutions, bioanalytical workflows can achieve greater efficiency, accuracy, and regulatory alignment, ultimately facilitating successful clinical development and approval.
| Graphical abstract. |
|---|
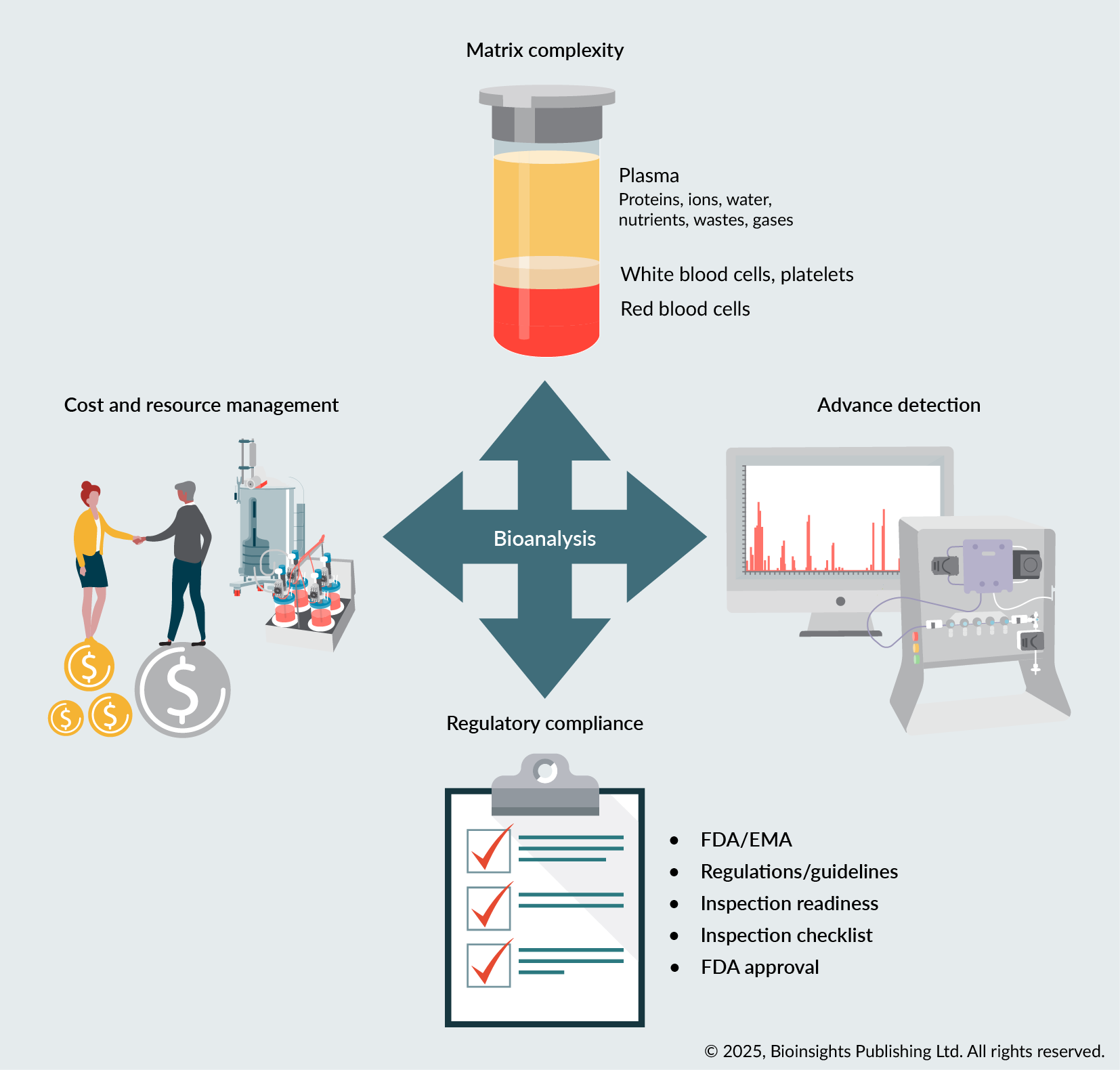 |
| Key challenges and strategic solutions in bioanalytical method development. This graphical abstract summarizes key challenges in bioanalytical method development—such as matrix complexity, sensitivity, regulatory compliance, and stability—and showcases strategic solutions like advanced sample prep, matrix-matched calibration, MRM, stability studies, and automation to enhance accuracy, reliability, and compliance. © 2025, BioInsights Publishing Ltd. All rights reserved |
