A framework for genomic integrity testing across the development lifecycle

Live30 webinars are thirty-minute presentations designed to update you on the latest innovations, applications, and data in a fast yet interactive format.
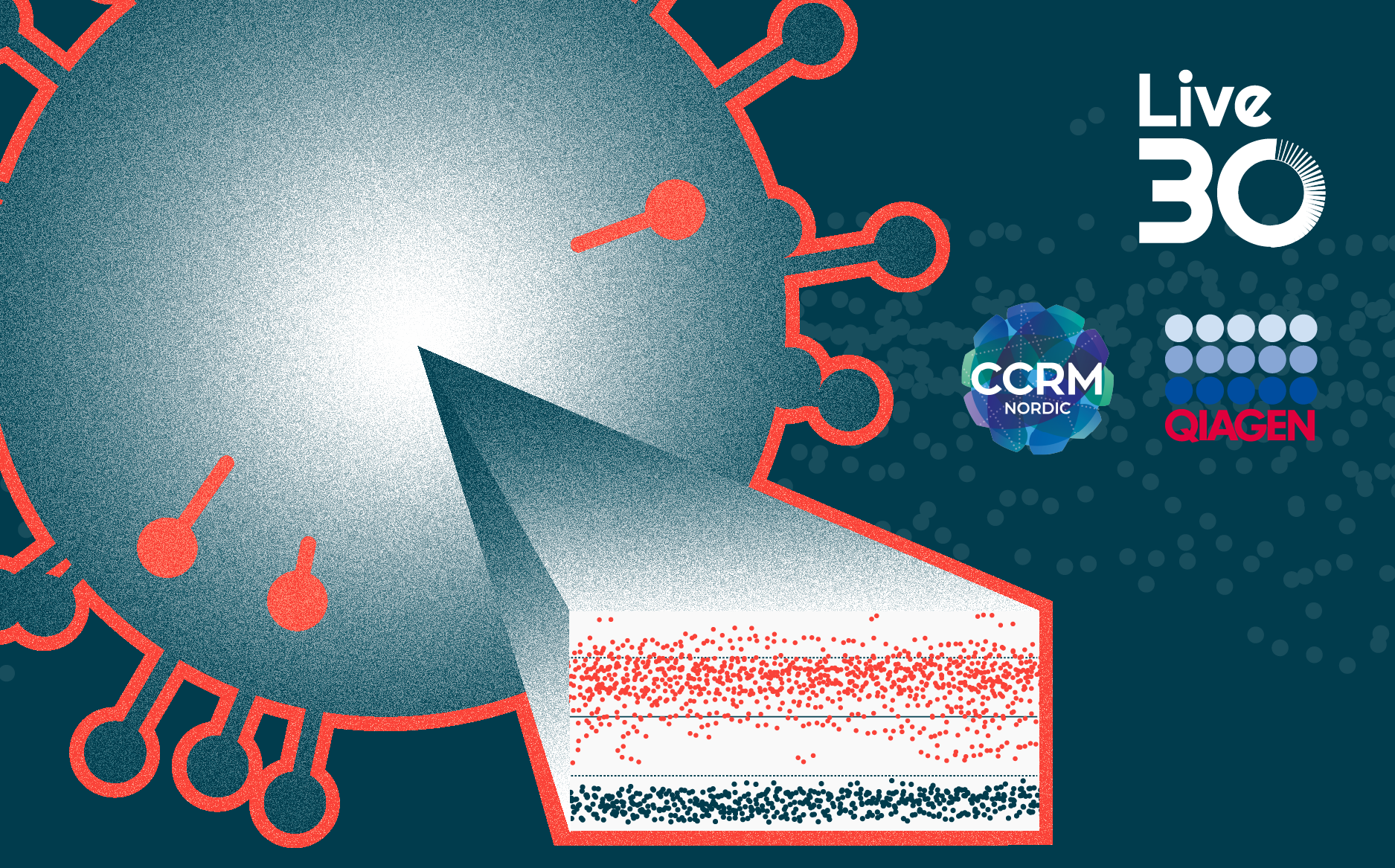
Genomic integrity is a critical focus in cell and gene therapy (CGT) development, as structural changes to the genome that arise during editing can lead to serious consequences like oncogenesis or clonal outgrowth. Regulatory agencies now emphasize the importance of assessing genomic integrity as part of the development process, with structural variant analysis increasingly being used across all development stages. Traditional cytogenetic tools like G-banding have limitations in detecting rare or complex events. Advanced cytogenetic tools that analyze hundreds of single cells can provide a more complete picture of genome stability, allowing detection of both rare and prevalent structural variants with greater confidence.
In this webinar, case studies will illustrate practical application of genomic integrity testing. The presentation includes an example involving CD33 CAR-NK cell therapy for AML. Structural variant testing was used to confirm transgene insertion accuracy, rule out off-target effects, and support the release of cells for patient use, highlighting how this type of testing informs both product safety and manufacturing quality.
- Learn how to integrate genomic integrity analysis—including chromosomal rearrangements, insertions, and transgene mapping into your QC workflows to meet evolving FDA guidance for CGTs
- Understand how detecting rare structural variants at the single-cell level can identify risks like clonal proliferation or insertional mutagenesis before they reach the clinic, to build safer, more robust therapeutic products
- Explore how cutting-edge cytogenetic tools, like those demonstrated in the AML CAR-NK case study, are setting new benchmarks for structural variant detection across therapy types and development phases
You have registered for this webinar
You might also like

Genomic integrity and safety assessment strategies for CRISPR-edited cell therapies
A science-driven framework for EV characterization to achieve clinical success
Exploring scalable, data-driven AAV manufacturing to achieve high yield and quality across serotypes